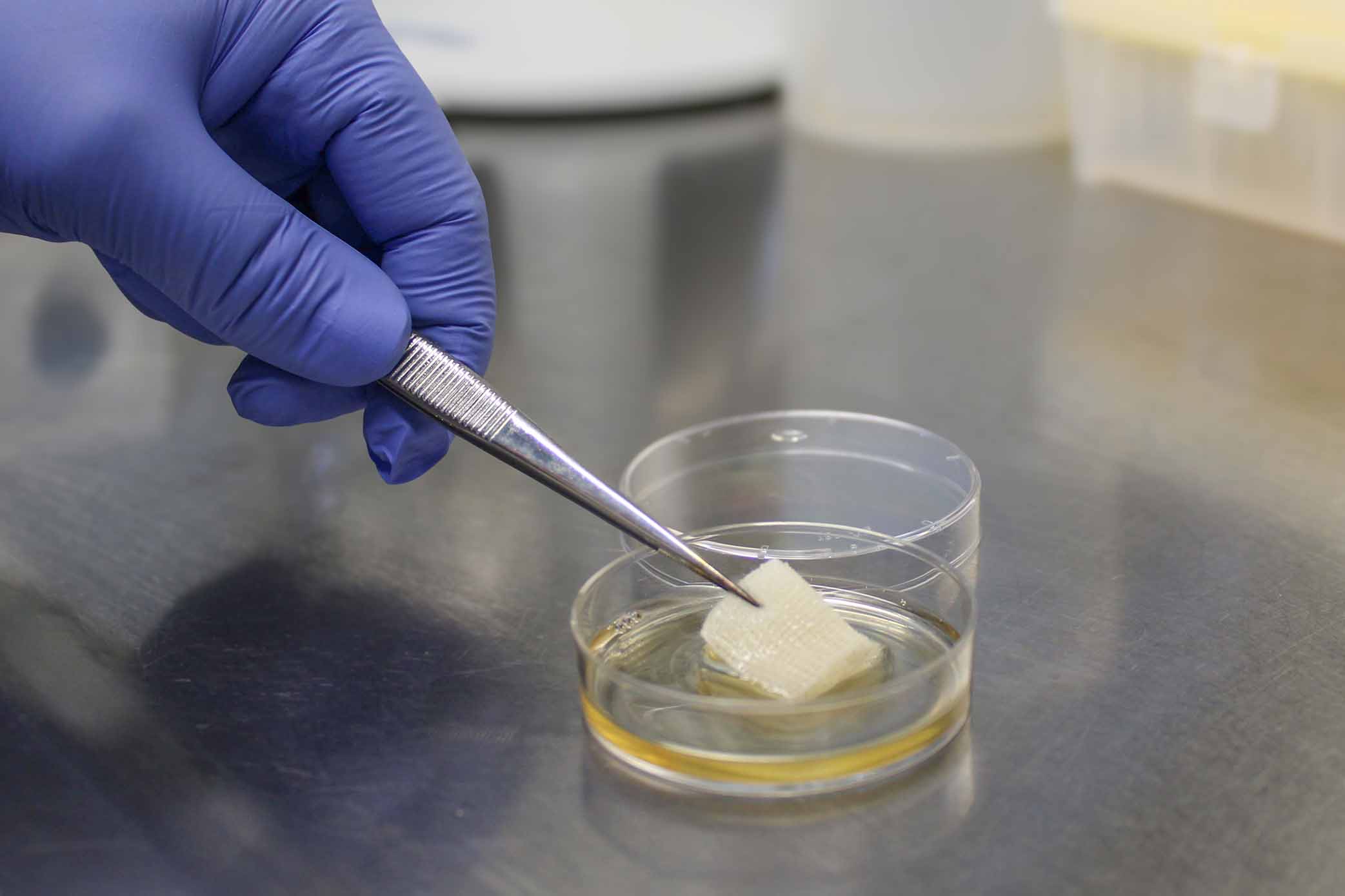
Shortly before the test, the saturation volume of a wound dressing is determined. (Image rights: OMPG / C. Reichmann)
A wide range of textiles, including wound dressings for medical use, can be tested using various standard procedures. “However, since the use of medical wound dressings places very specific demands on the material and antimicrobial treatment, it was rather difficult to carry out realistic testing using the standards available to date,” states Dr. Thomas Dauben, head of the OMPG biology laboratory. With the newly published DIN EN 17854 standard, which was created specifically for antimicrobial wound dressings, relevant factors such as nutrient-rich and serum-containing environmental conditions are now taken into account to a greater extent, according to Dauben. The standard has been available in Germany for the first time since October 2024, which means that there are now uniform specifications for testing these special medical devices throughout Europe.
Antimicrobial wound dressings are mainly used for infected wounds, as they can actively reduce the bacterial load and thus effectively promote wound healing thanks to a special coating. The test conditions specified in the DIN EN 17854 standard have been chosen so that the application can be simulated better than with previous test methods. “For example, testing is carried out against a broader spectrum of germs,” explains Dauben, referring to “gram-positive and gram-negative bacteria as well as yeasts.” In addition, a simulated wound fluid with a higher serum content is used in order to better replicate the environment of an infected wound. “This,” says Dauben, “enables a more reliable assessment of the effectiveness of antimicrobial wound dressings under simulated application conditions.”
Ideally, an antimicrobial wound dressing is compared with a neutral dressing for this test. The aim is to compare the number of viable microorganisms that can be removed from the various wound dressings after a defined incubation period. If a reduction in microorganisms can be detected on the treated wound dressing compared to the untreated version, it can be classified as microbiostatic or microbicidal, depending on the intensity of the reduction.
The OMPG biology lab team has been working intensively over the past few months to meet all the requirements for material testing in accordance with the new standard. “We are delighted to be able to offer flexible accreditation for the standard already,” says Dauben. This applies to general materials and products, but not to medical applications. In order to be able to test medical devices in an accredited manner, approval is now to be obtained in the near future from the German Accreditation Body (DAkkS) and the Central Office of the German States for Health Protection (ZLG).



![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/b/b/csm_brandpruefungen-und-elektroanwendungen_7036bf8d6b.jpg)

![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/8/a/csm_biologische-pruefungen_b330c70d45.jpg)


